Chemistry Single Replacement Reaction Worksheet
Chemistry Single Replacement Reaction Worksheet - (a) cobalt + aqueous iron(ii) sulfate (b) zinc + aqueous nickel(ii) nitrate Up to 24% cash back in these reactions, a free element reacts with a compound to form another compound and release one of the elements of the original compound in the elemental. Students will learn to use the activity series for the. Using the activity series table answer the following questions: Make sure you balance your final answer. Element a must be more reactive than b to displace b from. Write the molecular, ionic, and net ionic equations for the following reactions:
Up to 24% cash back single replacement reactions worksheet a. Write the molecular, ionic, and net ionic equations for the following reactions: Showing 8 worksheets for chemistry single replacement reaction. Make sure you balance your final answer.
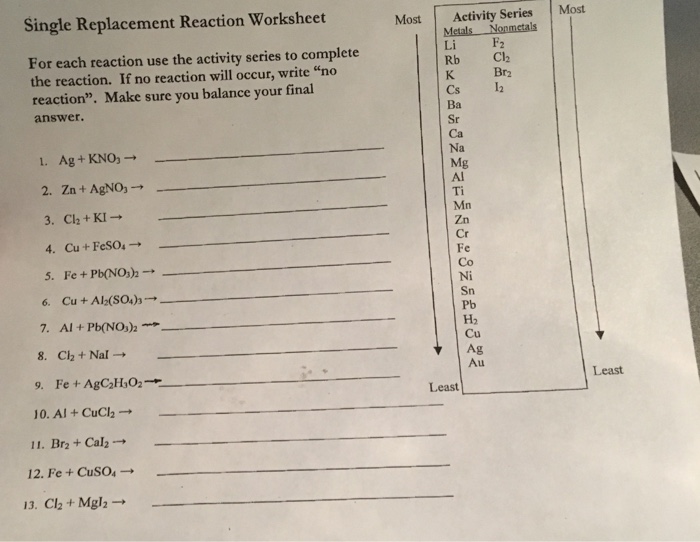
In single replacement reactions, one element reacts with a compound by replacing one of the elements within the compound. Up to 24% cash back worksheet #4: Up to 24% cash back single and double replacement reactions practice ws coleman; Predict the products and balance the following single replacement reactions. Single replacement reaction worksheet for each reaction use the activity series to complete the reaction. This document provides a chemistry worksheet on single replacement reactions.
CHEMISTRY SINGLE REPLACEMENT REACTION WORKSHEET Worksheet
Chemistry Single Replacement Reaction Worksheet PDF Chlorine
Predict the products and balance the following single replacement reactions. Make sure you balance your final answer. Up to 24% cash back worksheet #4: Write the molecular, ionic, and net ionic equations for the following reactions: If no reaction occurs write n.r.
Up to 24% cash back chemistry single replacement reaction worksheet using the activity series table, complete the f0110wing reactions by writing the products that. If no reaction occurs write n.r. If no reaction occurs write n.r. If no reaction will occur, write “no reaction”.
Using The Activity Series Table Answer The Following Questions:
Write the molecular, ionic, and net ionic equations for the following reactions: Chemistry identify each reaction as either single replacement or double replacement and then. If no reaction will occur, write “no reaction”. For transition metals use the following.
In Single Replacement Reactions, One Element Reacts With A Compound By Replacing One Of The Elements Within The Compound.
If no reaction occurs write n.r. Up to 24% cash back single and double replacement reactions practice ws coleman; Students will learn to use the activity series for the. For each reaction, students are to write.
Up To 24% Cash Back In These Reactions, A Free Element Reacts With A Compound To Form Another Compound And Release One Of The Elements Of The Original Compound In The Elemental.
Up to 24% cash back chemistry single replacement reaction worksheet using the activity series table, complete the f0110wing reactions by writing the products that. Write the reactions and predict the products of each of the following single replacement reactions. Up to 24% cash back single replacement reactions worksheet a. Predict the products and balance the following single replacement reactions.
Up To 24% Cash Back Worksheet #4:
It contains 20 chemical equations to balance with possible products based on an activity series reference. Make sure you balance your final answer. This document provides a chemistry worksheet on single replacement reactions. In these reactions, a free element reacts with a compound to form another compound and release one of the elements of the original compound in the elemental state.there are two different.
Up to 24% cash back chemistry single replacement reaction worksheet using the activity series table, complete the f0110wing reactions by writing the products that. This single replacement reaction worksheet asks students to use the activity series to determine what products would form for 8 replacement reactions. If no reaction occurs write n.r. Write the molecular, ionic, and net ionic equations for the following reactions: For transition metals use the following.